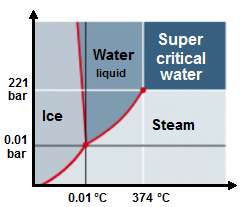
 A Super
Critical Water Oxidation reactor (SCWO) is driven by organic waste oxidized in super critical water. Water with a high content of
organic material is heated to about 400°C, the pressure is raised to 220
times higher than normal air pressure and oxygen is added, thereby all organic
material is oxidised to carbon dioxide, water and nitrogen gas (Gidner et al., 2000). The reactionen
requires at least 3 % organic material and genererates heat which preheat
incomming water and also produce steam. The process can be used for waste water treatment thereby the steam can produce electricity with an electric generator or used for driving a steam ship. The content of organic substance in the sewage can
be increased by adding grounded food waste or other biological material and
also oil. Additional fuel may be needed both for heating the reactor to working
temperature at start and to increase the organic substance to at least 3 %,
which makes equipment for dewatering unnessecary. If the content of organic substance in the water is to low,
oil can be injected into the reactor. The oil injection can be controlled
by the temperature. If temperature drops due to less organic substances in
the water, more oil is injected into the reactor.
A Super
Critical Water Oxidation reactor (SCWO) is driven by organic waste oxidized in super critical water. Water with a high content of
organic material is heated to about 400°C, the pressure is raised to 220
times higher than normal air pressure and oxygen is added, thereby all organic
material is oxidised to carbon dioxide, water and nitrogen gas (Gidner et al., 2000). The reactionen
requires at least 3 % organic material and genererates heat which preheat
incomming water and also produce steam. The process can be used for waste water treatment thereby the steam can produce electricity with an electric generator or used for driving a steam ship. The content of organic substance in the sewage can
be increased by adding grounded food waste or other biological material and
also oil. Additional fuel may be needed both for heating the reactor to working
temperature at start and to increase the organic substance to at least 3 %,
which makes equipment for dewatering unnessecary. If the content of organic substance in the water is to low,
oil can be injected into the reactor. The oil injection can be controlled
by the temperature. If temperature drops due to less organic substances in
the water, more oil is injected into the reactor.
Several substances have been found in outgoing wastewater that existing treatment processes can not remove. Examples include drug residues, micro plastics, persistent chemicals and pathogenic bacteria. Processes investigated to remove drug residues and micro pollutants is ozonation and activated carbon. Processes based on membrane technology can reduce the amount of micro plastics. Pathogenic bacteria can be killed with pulsed electric fields. A process based on oxidation in supercritical water, however, has the potential to remove all kinds of organic pollutants in wastewater. All drug residues, mickroplaster and organic pollutants in wastewater have been destroyed.
The nitrogen in the wastewater forms nitrogen gas in the reactor (http://aquarden.com/technology/scwo-systems/about-scwo/). If true, the future of wastewater a power plant with a SCWO reactor where the entire organic content in wastewater is converted into energy that can be used to drive an electrical generator. Since nitrogen compounds are converted to nitrogen gas, no energy consumning nitrogen removal that requires a carbon source (for denitrification) and energy for aeration (for nitrification) is needed. To counter global warming through emissions of carbon dioxide can the carbon dioxide formed is collected in tanks and delivered to another use. The phosphorus in the waste water can be separated from the inorganic residue. If no iron or aluminum salts are used, the removal of phosphorus is phosphate is more easily soluble phosphorus, which promotes phosphorus recycling.
Reactor corrosion and deposit problems have prevented commercialization of supercritical water oxidation for wastewater treatment (Xu et al., 2014; Chen et al., 2015; Kritzer & Dinjus, 2001). According to Kritzer and Dinjus (2001) is the problem to be solved mainly Corrosion problem especially in the heat exchanger (can be overcome by placing the pressure release before the heat exchanger) and that need of oxygen gas is makes the process expensive. Instead of buying oxygen solar cells and an electrolytic alkaline cell may be used.
At the anode, oxygen gas is produced: 2OH- --> ½ O2 + H2O + 2e-
At the cathode hydroxyl ions is produced from atmospheric oxygen: ½O2 + H2O + 2e- --> 2OH-
The cathode is separated from the anode with an anion selective membrane permeable to hydroxyl ions. To drive the reaction required energy can come from solar cells.
A research problem to be solved is new methods to obtain sufficiently high pressure in the reactor. In addition to the high pressure pump needful also a pressure reduction at the outlet. In the SCWO reactor used in Karlskoga the reactor was a long pipe and the pressure reduction a narrow capillary, located after the heat recovery with a heat exchanger. This required that the material to the reactor must be very finely ground. Chen et al. (2015) avoids clogging by using a reactor consisting of a chamber where the larger particles settle to the bottom and the effluent to the pressure reduction is taken from the top.
If a variable speed gear pump is used for effluent (flow-controlled pump) and a high pressure pump on the inflow, so the outflow from the reactor will be determined by the gear pump, the high pressure pump on the inflow will build up pressure in the reactor when it tries to squeeze more water in the reactor than what gear pump emits.
An alternative is that the SCWO reactor is designed as a cylinder in which inorganic particles settle to the bottom. The reactor is operated at 230 bar and the oxidation of organic material in the reactor gives a temperature of 600 °C. The effluent is through the reactor wall that is made of a porous material that reduces the pressure from 230 to 210 bar, which is 20 bar pressure reduction. Porosity and thickness of the wall must be adapted so that this pressure reduction is obtained. The water then transforms from the super critical phase to steam with a temperature of 600 °C and a pressure of 210 bar. The steam can be used to drive a steam engine/turbine constituting 90% of the pressure reduction after the reactor and a heat exchanger to preheat the inflow so that it becomes supercritical. Such a design would be more energy efficient compared to first take out the heat down to below 100 °C with heat exchanger and then reduce the pressure from 230 to 1 bar with a pressure reduction, which gives energy losses in both the heat exchanger and the pressure reduction.
If no oxygen is supplied to the reactor the organic material gasificicates instead of being oxidized (Qian et al., 2015). The transformation in Supercritical Water Gasification (SCWG) is equally complete. As no energy is produced in the reactor energy is needed to raise the pressure and the temperature to reach the supercritical phase.
Instead of oxygen, air can be supplied to the reactor. However, it requires a higher energy consumption because it more energy is needed to increase the pressure and compress a gas compared to the pressurizing a liquid (García-Rodríguez et al., 2015). When using air instead of oxygen also larger volumes of gas has to be forced into the reactor,
References
Chen, Zhong, Wang, Guangwei, Yin, Fengjun, Chen, Hongzhen & Xu, Yuanjian (2015) A new system design for supercritical water oxidation Chemical Engineering Journal 269: 343-351.
García-Rodríguez, Yoana, Mato, Fidel A., Martín, Alexandra, Bermejo, M. Dolores & Cocero, M. José (2015) Energy recovery from effluents of supercritical water oxidation reactors J. of Supercritical Fluids 104: 1–9.
Gidner A., Almemark* M., Stenmark L. och Ekengren* Ö. (2000). Treatment of sewage sludge by supercritical water oxidation. IBC´s 6th Annual Conference on Sludge. Feb. 16th-17th 2000, London, England.
Kritzer, Peter & Dinjus, Eckhard (2001) An assessment of supercritical water oxidation (SCWO) Existing problems, possible solutions and new reactor concepts Chemical Engineering Journal 83: 207–214.
Qian, Lili, Wang, Shuzhong, Xu, Donghai, Guo, Yang, Tang, Xingying & Wang, Longfei (2015) Treatment of sewage sludge in supercritical water and evaluation of the combined process of supercritical water gasification and oxidation Bioresource Technology 176: 218–224.
Xu, Donghai, Wang, Shuzhong, Huang, Chuanbao, Tang, Xingying & Guo, Yang (2014) Transpiring wall reactor in supercritical water oxidation Chemical engineering research and design 92: 2626–2639.
Mats Almemark* och Östen Ekengren*, works at IVL Swedish Environmental Research Institute and contribured to construct the pilot plant for SCWO which existed in Karlskoga, AquaCritox-processen developed by Chematur in Sweden in cooperation with IVL, which was later bought by CFI Group Ltd. Rubicon, Cork, Ireland http://www.scfi.eu/products/